Researchers at the Beckman Institute for Advanced Science and Technology developed an automated laboratory robot to run complex electrochemical experiments and analyze data.
With affordability and accessibility in mind, the researchers collaboratively created a benchtop robot that rapidly performs electrochemistry. Aptly named the Electrolab, this instrument greatly reduces the effort and time needed for electrochemical studies by automating many basic and repetitive laboratory tasks.
The Electrolab can be used to explore energy storage materials and chemical reactions that promote the use of alternative and renewable power sources like solar or wind energy, which are essential to combating climate change.
“We hope the Electrolab will allow new discoveries in energy storage while helping us share knowledge and data with other electrochemists — and non-electrochemists! We want them to be able to try things they couldn’t before,” said Joaquín Rodríguez-López, a professor in the Department of Chemistry at the University of Illinois Urbana-Champaign.
The interdisciplinary team was co-led by Rodríguez-López and Charles Schroeder, the James Economy professor in the Department of Materials Science and Engineering and a professor of chemical and biomolecular engineering at UIUC. Their work appears in the journal Device.
Electrochemistry is the study of electricity and its relation to chemistry. Chemical reactions release energy that can be converted into electricity — batteries used to power remote controllers or electric vehicles are perfect examples of this phenomenon.
In the opposite direction, electricity can also be used to drive chemical reactions. Electrochemistry can provide a green and sustainable alternative to many reactions that would otherwise require the use of harsh chemicals, and it can even drive chemical reactions that convert greenhouse gasses such as carbon dioxide into chemicals that are useful in other industries. These are relatively simple demonstrations of electrochemistry, but the growing demand to generate and store massive amounts of energy on a much larger scale is currently a prominent challenge.
One type of battery, known as a redox-flow battery, is used for grid-level storage and can store and bring power to entire electrical grids. The batteries explored by this collaboration use organic molecules to store energy and can be easily altered or tuned by changing the structure of those molecules. A major drawback of exploring redox-flow battery conditions is that it takes a lot of time and effort to identify a system that works, said Michael Pence, a graduate student of the Rodríguez-López Laboratory and a 2023 Beckman Institute Graduate Fellow.
The Electrolab started as an idea between Rodríguez-López and Schroeder based on collaborative project funded by the Joint Center for Energy Storage Research, an Energy Innovation Hub of the U.S. Department of Energy focused on advancing battery science and technology. Rodríguez-López and Schroeder put together an interdisciplinary team including programmers, engineers, and electrochemists. Initially, the idea was to create a microfabricated design, but the team decided to prioritize accessibility and transferability.
After establishing the final design of the Electrolab, the research group successfully created and tested an affordable device that is highly adaptable, made from common parts, and costs about $1,000 to build, which is key for its adoption by laboratories of all sizes. The team is openly sharing construction plans for this instrument, so that all researchers can benefit from it.
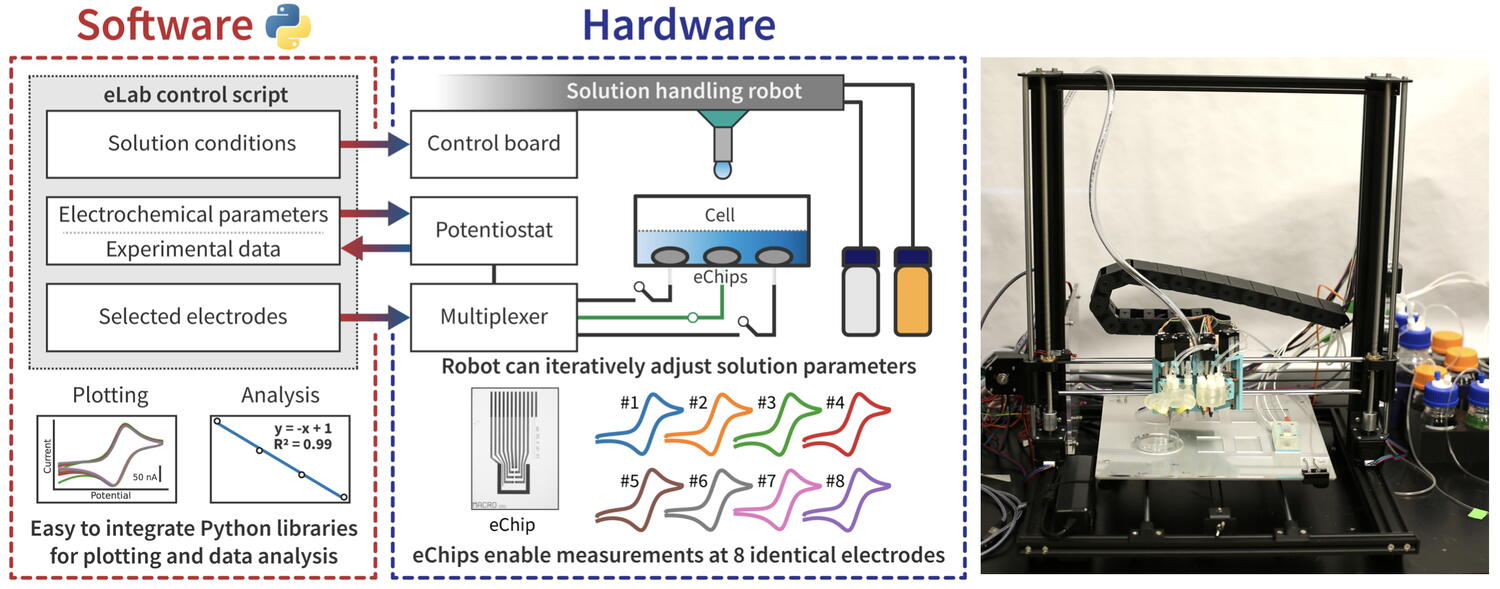
There are two main components of the Electrolab: hardware and software. The hardware consists of a standard 3D printer frame that was transformed into a solution-handling robot; microfabricated electrode arrays, or eChips; and electrochemical hardware. The frame allows the robot to move around within a designated area above electrochemical cells to dispense different liquids. The eChips measure electrical current which is necessary for understanding the electrochemical measurements.
The software component was created in Python (a free, open-source coding platform) that allows the user to connect with Electrolab to perform experiments. The software allows for fully automated data analysis, visual graphics, and plotting. When paired with machine learning, the Electrolab transforms from a robot completing predetermined tasks to a robot that can make decisions about the direction of the experiment while it is happening. Typically, an electrochemist handpicks datasets of interest to move forward, but the Electrolab uses the data it is collecting and analyzing in real time to make the next move. In other words, the Electrolab is making this science electro-fast.
The bottleneck of electrochemical characterization is the time required for in-depth analysis and characterization of new molecules and solutions. These are tasks like measuring voltages at which battery materials charge and discharge and figuring out the speed of side reactions. There are almost limitless ways to explore and tweak these systems but simply not enough time or bandwidth to explore every option.
The Electrolab is accelerating the discovery of new materials and will ultimately help combat climate change. Studying efficient energy conversion and exploring new energy storage materials used in redox-flow batteries would enable alternative energy sources like solar or wind energy to become more practical. At the heart of all that is electrochemistry, says Pence, and that is why Electrolab is so important.
In their recently published paper, the research team describes the Electrolab’s function in detail and report the findings of two experiments used to test the accuracy and robustness of their robot. The Electrolab performed more than 200 experiments across multiple conditions, analyzed the data, and even cleaned up after itself in 2 hours. This experiment would have taken 8 hours for the average electrochemist — depending on their caffeination level.
The second experiment tested the Electrolab’s ability to work as a specialist. Programmed to look at a next generation redox-flow battery material in a much more demanding type of experiment to find supporting electrolyte solutions, the Electrolab was modified with smaller, more sensitive electrodes and set to run entirely autonomously. It completed the tasks in less than four hours with no human interference, allowing researchers to work on other projects and reducing background noise that can often been seen in delicate electrochemical analyses.
Beyond exploring new battery materials, the Electrolab shows promise for exploring systems where electrochemistry is driving chemical reactions in a green and sustainable manner. As part of his Beckman research, Pence plans to use the Electrolab to screen conditions for oxidation of common biomass byproducts, finding ways to transform waste materials into value-added chemicals.
Left: Schematic diagram of the Electrolab's software and hardware components. Right: Photograph of the Electrolab. From "The Electrolab: An open source modular platform for automated characterization of redox-active electrolytes." by Oh et al. 2023, Oct 10. Device.
___________________________________________________________________________
Editor’s note:
The publication titled “The Electrolab: An open-source, modular platform for automated characterization of redox-active electrolytes” can be accessed online at https://doi.org/10.1016/j.device.2023.100103.
This research was financially supported by the Joint Center for Energy Storage Research, an Energy Innovation Hub funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences.
Media contact: Jenna Kurtzweil, kurtzwe2@illinois.edu